It's hard to say much of anything about particles if you don't know anything about the system they're in. When air is flowing through a tube, for instance, it's a constantly changing, turbulent mess. However, we can say a lot about systems that have reached equilibrium. Equilibrium is what happens when you leave the system sitting …
Category Archive: Thermo for Normals
Jul 08 2012
Thermo for Normals (part 18): Entropy
When we're talking thermo, we're talking about systems. A system could be the gas in a room, a chunk of metal, a beaker full of water---anything with a bunch of atoms. A system has volume, pressure, temperature, and internal energy. But, since we've started running into a problem with reversibility, you might start to wonder …
Jun 19 2012
Thermo for Normals (part 17): Reversibility and your car's engine
There are lots of processes in thermo that we can think of that are irreversible. That means that if you ran a film of the process backwards, what you see would never happen. When you put an ice cube into lukewarm tea, the ice cube melts. If you run the film backwards, you see a …
Jun 10 2012
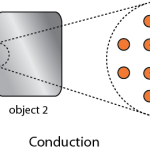
Thermo for Normals (part 16): How heat gets around
So far I've kind of just talked about heat moving from one system to another, without much regard to how that transfer actually happens. The way that heat actually gets around is hugely important, so we can't keep avoiding the issue. Of course, we know that heating is the transfer of energy from one set …
Jun 03 2012
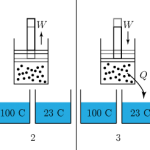
Thermo for Normals (part 15): Engines and the Second Law
Now that we know the 2nd law, let's look at some consequences. To review, the 2nd law says that there is no way to turn heat completely into work without changing something else. If we had a piston, and we heated it and let the gas inside it expand, it would do work pushing against …