The first and second laws of thermodynamics are basically simple, but there's so many consequences that you could spend a lifetime considering even all the ones you encounter every day. A lot of these also require a knowledge of properties of matter (phases, phase transitions, heat capacity, etc.). Here are a few questions to ponder, all of which are with everyday examples. I haven't covered any of these examples previously, but see if you can reason your way through.
- Can you freeze water with ice? What about with ice water?
- Metal tables feel cold to the touch, while wood tables don't. Why is that?
- While boiling water, about how long after you turn off the heat would you expect the water to stop boiling?
- Dumping salt onto icy roads makes them melt. How does that work?
If the answers don't come immediately, that's totally normal. None of these is straightforward, but all of them are commonplace. The world is full of incredibly complicated phenomena. That we can understand any of them is astounding.
Here are the answers as I would give them.
Can you freeze water with ice? What about with ice water?
To the former, the answer is a very qualified yes. You cannot use any old ice, and your chances of making this work are slim.
When an ice cube is put into contact with liquid water, the second law dictates that heat will flow from hot to cold. Heat will therefore move from the liquid into the solid, and if the ice is very cold (recall that ice can exist in principle down to absolute zero) then perhaps it can absorb this heat without melting. One would have to do this very slowly, so that the edges of the ice do not melt, and the heat has time to diffuse through the ice cube. Given enough time, heat could be transferred from the liquid to the ice until the liquid freezes.
Now, I said that you have to be careful not to let part of the ice melt. This is because ice water cannot freeze liquid water! The scenario depicted to the left in cartoon form is of a vial of water immersed in ice water, and the water in the vial will not freeze. How come?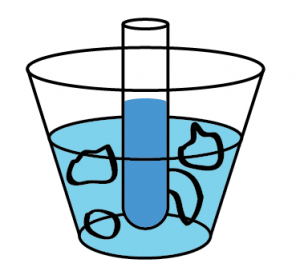 Well, if the ice won't freeze the water that's already in the cup, why would it freeze the other water? Ok, that's kind of a tricky answer. More explicitly, we can say that heat will only flow from the water in the vial to the ice water in the cup until the temperature of each is 32 fahrenheit, whereupon everything stops. The water in the vial and the water it is in contact in are at the same temperature, and heat only flows from hot to cold, not from ... same to same.
Well, if the ice won't freeze the water that's already in the cup, why would it freeze the other water? Ok, that's kind of a tricky answer. More explicitly, we can say that heat will only flow from the water in the vial to the ice water in the cup until the temperature of each is 32 fahrenheit, whereupon everything stops. The water in the vial and the water it is in contact in are at the same temperature, and heat only flows from hot to cold, not from ... same to same.
This might lead you to another question: then how the heck do ice cream makers work?
The more modern counter-top ice cream makers use a substance other than water to act as the heat sink. They can liquify while still remaining below the freezing point of water (or milk), and thus can keep absorbing heat.
Now, the old style of ice cream maker (which I had as a child) looks remarkably like my cartoon! It was literally just a rotating bucket of cream in an immersion of ice and water. Didn't we just say that can't work? Ah, but there's another trick here. The water in the immersion had to be salted for those machines to work. By putting salt into the water, the freezing point of water was suppressed, and so the liquid in the immersion was always colder than the cream. (That being said, these things took forever to work.)
Metal tables feel cold to the touch, while wood tables don't. Why is that?
Both tables are at room temperature, and skin is normally higher than that (unless it's a rather warm day). So, in both cases heat will flow from your hand into the table when you touch it. Since the metal table feels cold, we're left with two possibilities. The first is that the tables have vastly different heat capacities, so that a lot of heat can be transferred to the metal table without raising its temperature much, and thus continuing the transfer of heat, whereas the wood table heats up quickly in response to a bit of heat. This isn't true. Though the heat capacities are different, they are not very different.
The second possibility is that perhaps the metal table carries some of the heat to another part of the table, whereas the wood table isn't able to transfer the heat well. This is, of course, correct. Metal has vastly greater heat conductivity, meaning that heat transfer from your hand to the table is distributed throughout a large volume of solid, and thus increasing the amount of heat the table will take from your hand before it reaches a local temperature equal to that of your skin.
While boiling water, about how long after you turn off the heat would you expect the water to stop boiling?
Up to now, our sole understanding of a phase transition is that a substance at the transition temperature takes in heat and uses that heat, not to increase temperature, but rather to promote atoms from one phase to another. In this case, the stove puts heat into the water, and the water responds by ejecting some of its water molecules as gas (water vapor). If you stop the heating, then surely you stop the promotion of atoms to the gas. You might justly reason that the boiling should stop immediately.
Though this answer is basically right, things are not always quite so simple. One obvious problem is that the bottom of the pot is really fricken hot, as it was just in contact with the extremely hot stove. Until the heat from this metal has a chance to dissipate, likely some heat will continue entering the water, and some boiling will continue for a bit. Another complication is that some of the water in the pot may be at a temperature slightly above the boiling point, but is stuck somewhere beneath the surface and has not been able to form a bubble and percolate out. A sudden shock to the vessel could then cause the boiling to continue, though not for very long.
Dumping salt onto icy roads makes them melt. How does that work?
This certainly is a puzzle, isn't it? Most of us are likely aware that saltwater freezes at a lower temperature than pure water, and so if we could convert the ice to salt-water-ice, it may revert to liquid form. But that's not what's happening! We're putting two solids into contact and getting a liquid. You might counter that the salt may be at an elevated temperature from being on the truck, so some of the ice will melt from heat conduction, and combine with salt to make stable saltwater, but this leads us to the same issue: why does salt water in contact with ice (at the same temperature) somehow liquify the ice, which is still pure water?
The solution comes in the form of a somewhat bizarre concept of fluctuations. When we see ice floating in water, we imagine there is a boundary between the two phases: the surface of the ice cube. But the fact is that H2O molecules near the edge of the ice cube are jumping off all the time, and are replaced on average with liquid H2O molecules that run into the ice cube and attach. Though this is clearly not observable in such a large system, seeing it in a lab is not too difficult. The atoms are all jiggling, and random events like that happen constantly.
Fluctuations provide an entirely plausible route for the liquification of ice with exposure to salt. H2O molecules, or clusters of them, near the surface of the ice jump out and attach with NaCl, which lowers their freezing point. Without the salt, these molecules would normally just fluctuate back into the solid phase. However, because of the salt, suddenly these molecules are a lot less likely to go the backwards route in their fluctuation. Soon enough, all of the molecules have gone through this fluctuation, and what was once a sheet of ice is now a relatively harmless puddle of saltwater.
Closing thoughts
Though some are prone to describing the universe as amazingly simple, I think this is not the case. In fact, real life is amazingly complicated and subtle, full of pitfalls in thinking even for someone with a good grasp of fundamental and correct laws of nature. It is only the laws of nature that are simple; any given example is almost always a messy, imprecise, and speculative. Such is life.






1 comment
Rosaria Williams
5/9/2012 at 9:37 AM (UTC -5) Link to this comment
This is so cool! The closing thoughts, priceless! Rosaria