Now we move on to what is by far the most bizarre law of nature. The second law is frequently invoked, but it is by far the least well-understood. We start, as we did with the first law, with the caveman form:
If you put two things in contact in some way that are at different temperatures, heat will flow from the one with higher  to the one with lower
to the one with lower  until they are at the same temperature. It never goes the other way. There are actually some details about this that make the law very weird. But we'll have to come back to that later.
until they are at the same temperature. It never goes the other way. There are actually some details about this that make the law very weird. But we'll have to come back to that later.
Now, if we ever say something like "heat flows from hot to cold", we mean that two macroscopic (normal sized) objects of different temperatures will exchange internal energy when in contact. If I put two pieces of metal together that are at different temperatures, the atoms in the higher temperature object are jiggling more, and these collide with the slower jiggling atoms in the cold thing. Where's the law of nature? This is just Newtonian mechanics!
Thermodynamics is a statement about big things (lots of molecules). We use it when it's basically impossible to draw any conclusions from looking at Newton's laws. So this law is useful in telling you a very obvious fact without needing to think about the billions upon billions of atoms in the object.
In truth, the caveman version is only a part of the second law. You may have heard that this law is what makes a perpetual motion machine impossible. So we must think about that.
Now we're going to start talking about engines. An engine can be anything that absorbs heat, gives off heat, does work, has work done on it, and gathers or releases particles. A car engine does all of these things. An air conditioner does some of these, and so it is also an engine, as is a refrigerator. And, you are an engine, since you burn fuel (food) to make heat energy, and use that to do work moving your body around, pumping your blood, etc.
Here's the honest, complete second law.
(I know that this law seems to be very far removed from the caveman form. It's quite complicated to show that "heat flows from hot to cold" follows from this, and I'd rather leave it until later to draw the connection. For now, just keep both of these statements in mind.)
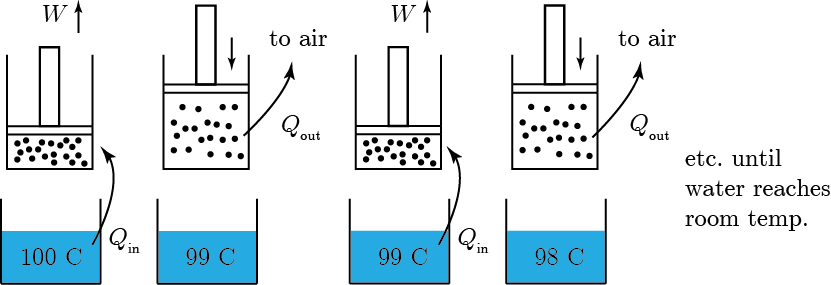
Let's make it very concrete. Suppose I have an engine that is just a piston with a gas inside of it, and I have a large vat of boiling water. You might think, based on the first law, that I could take all of the heat that the boiling water would lose in cooling to room temperature, and turn it all into work. I put the piston with gas in it in contact with the hot water, and heat flows into the gas. Then I let the piston expand and it does work (let's say it lifts up a weight). So far, so good. We haven't violated the law, because it says "without doing anything else", and we have done something else. We made our piston be extended. But, to get the piston back to its original state to use it again, we have to let it cool back down, which lets heat into the room that is waste. It also compresses the piston back to the original length. Now we can repeat for as many cycles until the water gets down to room temperature. At that point, we can't do any more. So we took some heat out of the hot bath, and we got some work out of it, but eventually we had to let some heat out somewhere cooler to get the machine reset.
There is actually another form of the 2nd law, which is equivalent in every way, but reads differently:
Your refrigerator is operating between two temperatures. The cold temperature inside the icebox, and the warm temperature outside (the room the fridge is in). To cool the inside, it takes in work from an electric motor and moves heat from the inside to the outside. The heat that gets thrown out to the room is not all waste heat; some of it was thermal energy inside the fridge, which we wanted to extract to make the air inside colder. However, the 2nd law says that the amount of heat thrown out is bigger than the work done. That is, some of the work we did was wasted, and showed up as heat. There is no way around it.
The second law has a number of important consequences. We'll open up that can of worms next.





